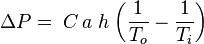Flue Gas Stack
A flue gas stack is a type of chimney, a vertical pipe, channel or similar structure through which combustion product gases called flue gases are exhausted to the outside air. Flue gases are produced when coal, oil, natural gas, wood or any other fuel is combusted in an industrial furnace, a power plant's steam-generating boiler, or other large combustion device. Flue gas is usually composed of carbon dioxide (CO2) and water vapor as well as nitrogen and excess oxygen remaining from the intake combustion air. It also contains a small percentage of pollutants such as particulate matter, carbon monoxide, nitrogen oxides and sulfur oxides. The flue gas stacks are often quite tall, up to 400 meters (1300 feet) or more, so as to disperse the exhaust pollutants over a greater area and thereby reduce the concentration of the pollutants to the levels required by governmental environmental policy and environmental regulation.
 When the flue gases are exhausted from stoves, ovens, fireplaces, or other small sources within residential abodes, restaurants, hotels, or other public buildings and small commercial enterprises, their flue gas stacks are referred to as chimneys.
When the flue gases are exhausted from stoves, ovens, fireplaces, or other small sources within residential abodes, restaurants, hotels, or other public buildings and small commercial enterprises, their flue gas stacks are referred to as chimneys.
Flue gas stack draft (or draught)
The combustion flue gases inside the flue gas stacks are much hotter than the ambient outside air and therefore less dense than the ambient air. That causes the bottom of the vertical column of hot flue gas to have a lower pressure than the pressure at the bottom of a corresponding column of outside air. That higher pressure outside the chimney is the driving force that moves the required combustion air into the combustion zone and also moves the flue gas up and out of the chimney. That movement or flow of combustion air and flue gas is called "natural draft (or draught)", "natural ventilation", "chimney effect", or "stack effect". The taller the stack, the more draft (or draught) is created.The equation below provides an approximation of the pressure difference, ΔP, (between the bottom and the top of the flue gas stack) that is created by the draft:
-
where: ΔP = available pressure difference, in Pa C = 0.0342 a = atmospheric pressure, in Pa h = height of the flue gas stack, in m To = absolute outside air temperature, in K Ti = absolute average temperature of the flue gas inside the stack, in K
The above equation is an approximation because it assumes that the molar mass of the flue gas and the outside air are equal and that the pressure drop through the flue gas stack is quite small. Both assumptions are fairly good but not exactly accurate.
The flue gas flow rate induced by the draft
As a "first guess" approximation, the following equation can be used to estimate the flue gas flow rate induced by the draft of a flue gas stack. The equation assumes that the molar mass of the flue gas and the outside air are equal and that the frictional resistance and heat losses are negligible:
| where: | |
| Q | = flue gas flow rate, m³/s |
|---|---|
| A | = cross-sectional area of chimney, m² (assuming it has a constant cross-section) |
| C | = discharge coefficient (usually taken to be from 0.65 to 0.70) |
| g | = gravitational acceleration at sea level, 9.807 m/s² |
| H | = height of chimney, m |
| Ti | = absolute average temperature of the flue gas in the stack, K |
| To | = absolute outside air temperature, K |
Designing chimneys and stacks to provide the correct amount of natural draft involves a great many factors such as:
- The height and diameter of the stack.
- The desired amount of excess combustion air needed to assure complete combustion.
- The temperature of the flue gases leaving the combustion zone.
- The composition of the combustion flue gas, which determines the flue gas density.
- The frictional resistance to the flow of the flue gases through the chimney or stack, which will vary with the materials used to construct the chimney or stack.
- The heat loss from the flue gases as they flow through the chimney or stack.
- The local atmospheric pressure of the ambient air, which is determined by the local elevation above sea level.
The calculation of many of the above design factors requires trial-and-error reiterative methods.
Governmental agencies in most countries have specific codes which govern how such design calculations must be performed. Many non-governmental organizations also have codes governing the design of chimneys and stacks (notably, the ASME codes).
Stack design
The design of large stacks poses considerable engineering challenges. Vortex shedding in high winds can cause dangerous oscillations in the stack, and may lead to its collapse. The use of helical faring is common to prevent this process occurring at or close to the resonant frequency of the stack.
Other items of interest
Some fuel-burning industrial equipment does not rely upon natural draft. Many such equipment items use large fans or blowers to accomplish the same objectives, namely: the flow of combustion air into the combustion chamber and the flow of the hot flue gas out of the chimney or stack.
A great many power plants are equipped with facilities for the removal of sulfur dioxide (i.e., flue gas desulfurization), nitrogen oxides (i.e, selective catalytic reduction, exhaust gas recirculation, thermal deNOx, or low NOx burners) and particulate matter (i.e., electrostatic precipitator)s. At such power plants, it is possible to use a cooling tower as a flue gas stack. Examples can be seen in Germany at the Power Station Staudinger Grosskrotzenburg and at the Rostock Power Station. Power plants without flue gas purification, would experience serious corrosion in such stacks.
In the United States and a number of other countries, atmospheric dispersion modeling studies are required to determine the flue gas stack height needed to comply with the local air pollution regulations. The United States also limits the maximum height of a flue gas stack to what is known as the "Good Engineering Practice (GEP)" stack height. In the case of existing flue gas stacks that exceed the GEP stack height, any air pollution dispersion modeling studies for such stacks must use the GEP stack height rather than the actual stack height.
Flue-gas desulfurization
Flue gas desulfurization (FGD) is a technology used to remove sulfur dioxide (SO2) from the exhaust flue gases of fossil fuel power plants. Fossil fuel power plants burn coal or oil to produce steam for steam turbines, which in turn drive electricity generators.
Sulfur dioxide is one of the elements forming acid rain. Tall flue gas stacks disperse emissions by diluting the pollutants in ambient air and transporting them to other regions.
As stringent environmental regulations regarding SO2 emissions have been enacted in many countries, SO2 is now being removed from flue gases by a variety of methods. The below is among the common methods used:
- Wet scrubbing using a slurry of alkaline sorbent, usually limestone or lime, or seawater to scrub gases;
- Spray-dry scrubbing using similar sorbent slurries;
- Wet sulfuric acid process recovering sulfur in the form of commercial quality sulfuric acid;
- SNOX Flue gas desulfurization removes sulfur dioxide, nitrogen oxides and particulates from flue gases;
- Dry sorbent injection systems.
For a typical coal-fired power station, FGD will remove 95 percent or more of the SO2 in the flue gases.
History
 Methods of removing sulfur dioxide from boiler and furnace exhaust gases have been studied for over 150 years. Early ideas for flue gas desulfurization were established in England around 1850.
Methods of removing sulfur dioxide from boiler and furnace exhaust gases have been studied for over 150 years. Early ideas for flue gas desulfurization were established in England around 1850.
With the construction of large scale power plants in England in the 1920s, the problems associated with large volumes of SO2 from a single site began to concern the public. The SO2 emissions problem did not receive much attention until 1929, when the House of Lords upheld the claim of a landowner against the Barton Electricity Works of the Manchester Corporation for damages to his land resulting from SO2 emissions. Shortly thereafter, a press campaign was launched against the erection of power plants within the confines of London. This outcry led to the imposition of SO2 controls on all such power plants.
The first major FGD unit at a utility was installed in 1931 at Battersea Station, owned by London Power Company. In 1935, an FGD system similar to that installed at Battersea went into service at Swansea Power Station. The third major FGD system was installed in 1938 at Fulham Power Station. These three early large-scale FGD installations were abandoned during World War II. Large-scale FGD units did not reappear at utilities until the 1970s, where most of the installations occurred in the United States and Japan.
As of June 1973, there were 42 FGD units in operation, 36 in Japan and 6 in the United States, ranging in capacity from 5 MW to 250 MW. As of around 1999 and 2000, FGD units were being used in 27 countries, and there were 678 FGD units operating at a total power plant capacity of about 229 gigawatts. About 45% of the FGD capacity was in the U.S., 24% in Germany, 11% in Japan, and 20% in various other countries. Approximately 79% of the units, representing about 199 gigawatts of capacity, were using lime or limestone wet scrubbing. About 18% (or 25 gigawatts) utilized spray-dry scrubbers or sorbent injection systems.
Sulfuric acid mist formation
Fossil fuels such as coal and oil contain a significant amount of sulfur. When fossil fuels are burned, about 95 percent or more of the sulfur is generally converted to sulfur dioxide (SO2). Such conversion happens under normal conditions of temperature and of oxygen present in the flue gas. However, there are circumstances under which such reaction may not occur.
For example, when the flue gas has too much oxygen and the SO2 is further oxidized to sulfur trioxide (SO3). Actually, too much oxygen is only one of the ways that SO3 is formed. Gas temperature is also an important factor. At about 800 °C, formation of SO3 is favored. Another way that SO3 can be formed is through catalysis by metals in the fuel. Such reaction is particularly true for heavy fuel oil, where a significant amount of vanadium is present. In whatever way SO3 is formed, it does not behave like SO2 in that it forms a liquid aerosol known as sulfuric acid (H2SO4) mist that is very difficult to remove. Generally, about 1% of the sulfur dioxide will be converted to SO3. Sulfuric acid mist is often the cause of the blue haze that often appears as the flue gas plume dissipates. Increasingly, this problem is being addressed by the use of wet electrostatic precipitators.
FGD chemistry
Basic principles
Most FGD systems employ two stages: one for fly ash removal and the other for SO2 removal. Attempts have been made to remove both the fly ash and SO2 in one scrubbing vessel. However, these systems experienced severe maintenance problems and low removal efficiency. In wet scrubbing systems, the flue gas normally passes first through a fly ash removal device, either an electrostatic precipitator or a wet scrubber, and then into the SO2 absorber. However, in dry injection or spray drying operations, the SO2 is first reacted with the sorbent and then the flue gas passes through a particulate control device.
Another important design consideration associated with wet FGD systems is that the flue gas exiting the absorber is saturated with water and still contains some SO2. These gases are highly corrosive to any downstream equipment such as fans, ducts, and stacks. Two methods that can minimize corrosion are: (1) reheating the gases to above their dew point, or (2) choosing construction materials and design conditions that allow equipment to withstand the corrosive conditions. Both alternatives are expensive, and engineers designing the system determine which method to use on a site-by-site basis.
Scrubbing with a basic solid or solution
SO2 is an acid gas and thus the typical sorbent slurries or other materials used to remove the SO2 from the flue gases are alkaline. The reaction taking place in wet scrubbing using a CaCO3 (limestone) slurry produces CaSO3 (calcium sulfite) and can be expressed as:
- CaCO3 (solid) + SO2 (gas) → CaSO3 (solid) + CO2 (gas)
When wet scrubbing with a Ca(OH)2 (lime) slurry, the reaction also produces CaSO3 (calcium sulfite) and can be expressed as:
- Ca(OH)2 (solid) + SO2 (gas) → CaSO3 (solid) + H2O (liquid)
When wet scrubbing with a Mg(OH)2 (magnesium hydroxide) slurry, the reaction produces MgSO3 (magnesium sulfite) and can be expressed as:
- Mg(OH)2 (solid) + SO2 (gas) → MgSO3 (solid) + H2O (liquid)
To partially offset the cost of the FGD installation, in some designs, the CaSO3 (calcium sulfite) is further oxidized to produce marketable CaSO4 · 2H2O (gypsum). This technique is also known as forced oxidation:
- CaSO3 (solid) + H2O (liquid) + ½O2 (gas) → CaSO4 (solid) + H2O
A natural alkaline usable to absorb SO2 is seawater. The SO2 is absorbed in the water, and when oxygen is added reacts to form sulfate ions SO4- and free H+. The surplus of H+ is offset by the carbonates in seawater pushing the carbonate equilibrium to release CO2 gas:
- SO2 (gas) + H2O + ½O2 (gas)→ SO42- (solid) + 2H+
- HCO3- + H+ → H2O + CO2 (gas)
Types of wet scrubbers used in FGD
To promote maximum gas-liquid surface area and residence time, a number of wet scrubber designs have been used, including spray towers, venturis, plate towers, and mobile packed beds. Because of scale buildup, plugging, or erosion, which affect FGD dependability and absorber efficiency, the trend is to use simple scrubbers such as spray towers instead of more complicated ones. The configuration of the tower may be vertical or horizontal, and flue gas can flow cocurrently, countercurrently, or crosscurrently with respect to the liquid. The chief drawback of spray towers is that they require a higher liquid-to-gas ratio requirement for equivalent SO2 removal than other absorber designs.
Mobile-bed scrubbers
Venturi-rod scrubbers
A venturi scrubber is a converging/diverging section of duct. The converging section accelerates the gas stream to high velocity. When the liquid stream is injected at the throat, which is the point of maximum velocity, the turbulence caused by the high gas velocity atomizes the liquid into small droplets, which creates the surface area necessary for mass transfer to take place. The higher the pressure drop in the venturi, the smaller the droplets and the higher the surface area. The penalty is in power consumption.
For simultaneous removal of SO2 and fly ash, venturi scrubbers can be used. In fact, many of the industrial sodium-based throwaway systems are venturi scrubbers originally designed to remove particulate matter. These units were slightly modified to inject a sodium-based scrubbing liquor. Although removal of both particles and SO2 in one vessel can be economic, the problems of high pressure drops and finding a scrubbing medium to remove heavy loadings of fly ash must be considered. However, in cases where the particle concentration is low, such as from oil-fired units, it can be more effective to remove particulate and SO2 simultaneously.
Plate towers
Packed bed scrubbers
A packed scrubber consists of a tower with packing material inside. This packing material can be in the shape of saddles, rings, or some highly specialized shapes designed to maximize contact area between the dirty gas and liquid. Packed towers typically operate at much lower pressure drops than venturi scrubbers and are therefore cheaper to operate. They also typically offer higher SO2 removal efficiency. The drawback is that they have a greater tendency to plug up if particles are present in excess in the exhaust air stream.
Spray towers
A spray tower is the simplest type of scrubber. It consists of a tower with spray nozzles, which generate the droplets for surface contact. Spray towers are typically used when circulating a slurry (see below). The high speed of a venturi would cause erosion problems, while a packed tower would plug up if it tried to circulate a slurry.
Counter-current packed towers are infrequently used because they have a tendency to become plugged by collected particles or to scale when lime or limestone scrubbing slurries are used.
Scrubbing reagent
As explained above, alkaline sorbents are used for scrubbing flue gases to remove SO2. Depending on the application, the two most important are lime and sodium hydroxide (also known as caustic soda). Lime is typically used on large coal or oil fired boilers as found in power plants, as it is very much less expensive than caustic soda. The problem is that it results in a slurry being circulated through the scrubber instead of a solution. This makes it harder on the equipment. A spray tower is typically used for this application. The use of lime results in a slurry of calcium sulfite (CaSO3) that must be disposed of. Fortunately, calcium sulfite can be oxidized to produce by-product gypsum (CaSO4 · 2H2O) which is marketable for use in the building products industry.
Caustic soda is limited to smaller combustion units because it is more expensive than lime, but it has the advantage that it forms a solution rather than a slurry. This makes it easier to operate. It produces a solution of sodium sulfite/bisulfite (depending on the pH), or sodium sulfate that must be disposed of. This is not a problem in a kraft pulp mill for example, where this can be a source of makeup chemicals to the recovery cycle.
Scrubbing with sodium sulfite solution
It is possible to scrub sulfur dioxide by using a cold solution of sodium sulfite, this forms a sodium hydrogen sulfite solution. By heating this solution it is possible to reverse the reaction to form sulfur dioxide and the sodium sulfite solution.
In some ways this can be thought of as being similar to the reversible liquid-liquid extraction of an inert gas such as xenon or radon (or some other solute which does not undergo a chemical change during the extraction) from water to another phase. While a chemical change does occur during the extraction of the sulfur dioxide from the gas mixture, it is the case that the extraction equilibrium is shifted by changing the temperature rather than by the use of a chemical reagent.
Gas phase oxidation followed by reaction with ammonia
A new, emerging flue gas desulfurization technology has been described by the IAEA. It is a radiation technology where an intense beam of electrons is fired into the flue gas at the same time as ammonia is added to the gas. The Chendu power plant in China started up such a flue gas desulfurization unit on a 100 MW scale in 1998. The Pomorzany power plant in Poland also started up a similar sized unit in 2003 and that plant removes both sulfur and nitrogen oxides. Both plants are reported to be operating successfully. However, the accelerator design principles and manufacturing quality need further improvement for continuous operation in industrial conditions.
No radioactivity is required or created in the process. The electron beam is generated by a device similar to the electron gun in a TV set. This device is called an accelerator. This is an example of a radiation chemistry process where the physical effects of radiation are used to process a substance.
The action of the electron beam is to promote the oxidation of sulfur dioxide to sulfur(VI) compounds. The ammonia reacts with the sulfur compounds thus formed to produce ammonium sulfate which can be used as a fertilizer according to the IAEA. In addition, it can be used to lower the nitrogen oxide content of the flue gas. This method has attained industrial plant scale.
Facts and statistics
Flue gas desulfurization scrubbers have been applied to combustion units firing coal and oil that range in size from 5 MW to 1500 MW. Scottish Power are spending £400 million installing FGD at Longannet power station which has a capacity of over 2 GW. Dry scrubbers and spray scrubbers have generally been applied to units smaller than 300 MW.
Approximately 85% of the flue gas desulfurization units installed in the US are wet scrubbers, 12% are spray dry systems and 3% are dry injection systems.
The highest SO2 removal efficiencies (greater than 90%) are achieved by wet scrubbers and the lowest (less than 80%) by dry scrubbers. However, the newer designs for dry scrubbers are capable of achieving efficiencies in the order of 90%.
In spray drying and dry injection systems, the flue gas must first be cooled to about 10-20 °C above adiabatic saturation to avoid wet solids deposition on downstream equipment and plugging of baghouses.
The capital, operating and maintenance costs per short ton of SO2 removed (in 2001 US dollars) are:
- For wet scrubbers larger than 400 MW, the cost is $200 to $500 per ton
- For wet scrubbers smaller than 400 MW, the cost is $500 to $5,000 per ton
- For spray dry scrubbers larger than 200 MW, the cost is $150 to $300 per ton
- For spray dry scrubbers smaller than 200 MW, the cost is $500 to $4,000 per ton
Alternative methods of reducing sulfur dioxide emissions
An alternative to removing sulfur from the flue gases after burning is to remove the sulfur from the fuel before or during combustion. Hydrodesulfurization of fuel has been used for treating fuel oils before use. Fluidized bed combustion adds lime to the fuel during combustion. The lime reacts with the SO2 to form sulfates which become part of the ash.
Electricity Generation
Electricity generation is the process of creating electricity from other forms of energy.
The fundamental principles of electricity generation were discovered during the 1820s and early 1830s by the British scientist Michael Faraday. His basic method is still used today: electricity is generated by the movement of a loop of wire, or disc of copper between the poles of a magnet.
For electric utilities, it is the first process in the delivery of electricity to consumers. The other processes, electric power transmission, electricity distribution, and electrical power storage and recovery using pumped storage methods are normally carried out by the electrical power industry.
Electricity is most often generated at a power station by electromechanical generators, primarily driven by heat engines fueled by chemical combustion or nuclear fission but also by other means such as the kinetic energy of flowing water and wind. There are many other technologies that can be and are used to generate electricity such as solar photovoltaics and geothermal power.
There are seven fundamental methods of directly transforming other forms of energy into electrical energy:
- Static electricity, from the physical separation and transport of charge (examples: triboelectric effect and lightning)
- Electromagnetic induction, where an electrical generator, dynamo or alternator transforms kinetic energy (energy of motion) into electricity
- Electrochemistry, the direct transformation of chemical energy into electricity, as in a battery, fuel cell or nerve impulse
- Photoelectric effect, the transformation of light into electrical energy, as in solar cells
- Thermoelectric effect, direct conversion of temperature differences to electricity, as in thermocouples, thermopiles, and Thermionic converters.
- Piezoelectric effect, from the mechanical strain of electrically anisotropic molecules or crystals
- Nuclear transformation, the creation and acceleration of charged particles (examples: betavoltaics or alpha particle emission)
Static electricity was the first form discovered and investigated, and the electrostatic generator is still used even in modern devices such as the Van de Graaff generator and MHD generators. Electrons are mechanically separated and transported to increase their electric potential.
Almost all commercial electrical generation is done using electromagnetic induction, in which mechanical energy forces an electrical generator to rotate. There are many different methods of developing the mechanical energy, including heat engines, hydro, wind and tidal power.
The direct conversion of nuclear energy to electricity by beta decay is used only on a small scale. In a full-size nuclear power plant, the heat of a nuclear reaction is used to run a heat engine. This drives a generator, which converts mechanical energy into electricity by magnetic induction.
Most electric generation is driven by heat engines. The combustion of fossil fuels supplies most of the heat to these engines, with a significant fraction from nuclear fission and some from renewable sources. The modern steam turbine invented by Sir Charles Parsons in 1884 - today generates about 80 percent of the electric power in the world using a variety of heat sources.
Turbines
All turbines are driven by a fluid acting as an intermediate energy carrier. Many of the heat engines just mentioned are turbines. Other types of turbines can be driven by wind or falling water.Sources include:
- Steam - Water is boiled by:
- Nuclear fission,
- The burning of fossil fuels (coal, natural gas, or petroleum). In hot gas (gas turbine), turbines are driven directly by gases produced by the combustion of natural gas or oil. Combined cycle gas turbine plants are driven by both steam and natural gas. They generate power by burning natural gas in a gas turbine and use residual heat to generate additional electricity from steam. These plants offer efficiencies of up to 60%.
- Renewables. The steam generated by:
- Biomass
- The sun as the heat source: solar parabolic troughs and solar power towers concentrate sunlight to heat a heat transfer fluid, which is then used to produce steam.
- Geothermal power. Either steam under pressure emerges from the ground and drives a turbine or hot water evaporates a low boiling liquid to create vapour to drive a turbine.
- Other renewable sources:
- Water (hydroelectric) - Turbine blades are acted upon by flowing water, produced by hydroelectric dams or tidal forces.
- Wind - Most wind turbines generate electricity from naturally occurring wind. Solar updraft towers use wind that is artificially produced inside the chimney by heating it with sunlight, and are more properly seen as forms of solar thermal energy.
Reciprocating engines
Small electricity generators are often powered by reciprocating engines burning diesel, biogas or natural gas. Diesel engines are often used for back up generation, usually at low voltages. However most large power grids also use diesel generators, originally provided as emergency back up for a specific facility such as a hospital, to feed power into the grid during certain circumstances. Biogas is often combusted where it is produced, such as a landfill or wastewater treatment plant, with a reciprocating engine or a microturbine, which is a small gas turbine.
Photovoltaic panels
Unlike the solar heat concentrators mentioned above, photovoltaic panels convert sunlight directly to electricity. Although sunlight is free and abundant, solar electricity is still usually more expensive to produce than large-scale mechanically generated power due to the cost of the panels. Low-efficiency silicon solar cells have been decreasing in cost and multijunction cells with close to 30% conversion efficiency are now commercially available. Over 40% efficiency has been demonstrated in experimental systems. Until recently, photovoltaics were most commonly used in remote sites where there is no access to a commercial power grid, or as a supplemental electricity source for individual homes and businesses. Recent advances in manufacturing efficiency and photovoltaic technology, combined with subsidies driven by environmental concerns, have dramatically accelerated the deployment of solar panels. Installed capacity is growing by 40% per year led by increases in Germany, Japan, California and New Jersey.
Other generation methods
Various other technologies have been studied and developed for power generation. Solid-state generation (without moving parts) is of particular interest in portable applications. This area is largely dominated by thermoelectric (TE) devices, though thermionic (TI) and thermophotovoltaic (TPV) systems have been developed as well. Typically, TE devices are used at lower temperatures than TI and TPV systems. Piezoelectric devices are used for power generation from mechanical strain, particularly in power harvesting. Betavoltaics are another type of solid-state power generator which produces electricity from radioactive decay. Fluid-based magnetohydrodynamic (MHD) power generation has been studied as a method for extracting electrical power from nuclear reactors and also from more conventional fuel combustion systems. Osmotic power finally is another possibility at places where salt and sweet water merges.
Electrochemical electricity generation is also important in portable and mobile applications. Currently, most electrochemical power comes from closed electrochemical cells ("batteries"), which are arguably utilized more as storage systems than generation systems, but open electrochemical systems, known as fuel cells, have been undergoing a great deal of research and development in the last few years. Fuel cells can be used to extract power either from natural fuels or from synthesized fuels (mainly electrolytic hydrogen) and so can be viewed as either generation systems or storage systems depending on their use.